Dược phẩm có nguồn gốc thực vật là một lĩnh vực nghiên cứu đầy hứa hẹn và tiếp tục thu hút sự quan tâm đáng kể từ các quốc gia, khu vực, cộng đồng khoa học và các công ty dược phẩm trên toàn thế giới. Trong số đó, α- và β-amyrin đã được xác định là các hợp chất triterpenoid có giá trị cao với phổ rộng các đặc tính trị liệu tiềm năng, bao gồm hoạt tính chống viêm, chống tiểu đường, chống xơ vữa động mạch, giảm đau, chống bệnh gút, bảo vệ thần kinh, chống bệnh Parkinson, chống ung thư, kháng khuẩn và chống HIV. Thông tin và dữ liệu liên quan đã được thu thập thông qua việc tìm kiếm toàn diện trong các cơ sở dữ liệu khoa học chính, bao gồm Web of Science, Elsevier và Thư viện Y khoa Quốc gia. Nghiên cứu này nhấn mạnh tiềm năng dược phẩm của α- và β-amyrin, được hỗ trợ bởi bằng chứng cụ thể từ các thử nghiệm in vivo, in vitro và lâm sàng. Các phương pháp chiết xuất khác nhau đối với α- và β-amyrin được thảo luận, tiếp theo là các khuyến nghị về hướng phát triển trong tương lai của các hợp chất này như các tác nhân dược phẩm và thành phần thực phẩm chức năng. Bài đánh giá này nêu bật vai trò trị liệu của các hợp chất α- và β-amyrin trong việc phòng ngừa và điều trị nhiều bệnh nghiêm trọng trên toàn thế giới, có khả năng mở ra những cơ hội và hướng đi mới cho ngành công nghiệp dược phẩm.
Từ khóa:α- và β-amyrin ; chống viêm ; chống tiểu đường ; chống xơ vữa động mạch; giảm đau ; chống bệnh gút; tác dụng tích cực lên hệ thần kinh ; chống bệnh Parkinson ; chống ung thư ; kháng khuẩn ; chống HIV
1. Giới thiệu
Dược phẩm là mặt hàng có ý nghĩa xã hội cao [ 1 ] và là nhu cầu thiết yếu của thế giới [ 2 ]. Vai trò của dược phẩm trong điều trị, phòng ngừa và cải thiện sức khỏe, kéo dài và nâng cao chất lượng cuộc sống của hàng tỷ người trên toàn thế giới là không thể phủ nhận [ 3 , 4 ]. Nhu cầu toàn cầu về dược phẩm đang tăng nhanh [ 5 ]. Nhu cầu toàn cầu về dược phẩm đang tăng nhanh, đòi hỏi phải mở rộng và nâng cao năng lực sản xuất [ 6 ]. Thị trường thuốc thảo dược toàn cầu được định giá khoảng 230,03 tỷ USD vào năm 2021 và dự kiến sẽ tăng lên 430,05 tỷ USD vào năm 2028, phản ánh tốc độ tăng trưởng kép hàng năm (CAGR) là 11,32% [ 7 ].
Sự gia tăng nhanh chóng của dân số toàn cầu, cùng với sự gia tăng ô nhiễm môi trường, đã thúc đẩy nhu cầu dược phẩm tăng cao và ảnh hưởng sâu sắc đến mô hình tiêu thụ dược phẩm trên toàn thế giới [ 8 ]. Trong những thập kỷ gần đây, nghiên cứu và phát triển dược phẩm đã nhận được sự quan tâm đáng kể từ các chính phủ, nhà khoa học, công ty, cá nhân và tổ chức trên toàn thế giới [ 9 ]. Theo các tiêu chuẩn toàn cầu để đánh giá điều kiện sống, việc tiếp cận thuốc men để chăm sóc sức khỏe được coi là một tiêu chuẩn quan trọng. Vì lý do này, việc phát triển dược phẩm luôn được các nhà lãnh đạo thế giới ưu tiên như một yếu tố quan trọng của cả an sinh xã hội và phát triển quốc gia [ 10 ]. Sau đại dịch COVID-19 và những dự đoán về các thảm họa tự nhiên trong tương lai có thể dẫn đến sự xuất hiện của các bệnh mới, vai trò và tầm quan trọng của ngành công nghiệp dược phẩm đã trở nên quan trọng hơn bao giờ hết. Kết quả là, lĩnh vực này ngày càng thu hút các nghiên cứu khoa học tập trung vào việc phân tích các biến thể virus và mầm bệnh để phát triển các chiến lược hiệu quả nhằm chống lại các bệnh nguy hiểm và phức tạp hơn [ 11 ]. Nếu vị thế của ngành công nghiệp dược phẩm bị lung lay, nó có thể dẫn đến tình trạng thiếu thuốc thiết yếu, gây ra lo lắng và gián đoạn trên diện rộng trong xã hội, và có khả năng ảnh hưởng đến sự ổn định chính trị – xã hội [ 12 ]. Hơn nữa, trong bối cảnh dân số tăng nhanh, xung đột dai dẳng và sự xuất hiện của dịch bệnh, nhu cầu về thuốc tiếp tục leo thang, làm phức tạp thêm bối cảnh chăm sóc sức khỏe toàn cầu [ 13 ]. Có một xu hướng toàn cầu ngày càng tăng hướng tới việc sử dụng các sản phẩm tự nhiên như nguyên liệu thô trong sản xuất thuốc, thực phẩm chức năng, mỹ phẩm, thực phẩm chức năng, sản phẩm dinh dưỡng và đồ uống thảo dược, tất cả đều được thiết kế để thúc đẩy và bảo vệ sức khỏe con người [ 14 ].
Triterpenoid là một nhóm terpen bao gồm sáu đơn vị isopren với công thức phân tử C30H48 . Chúng cũng có thể được coi là bao gồm ba đơn vị terpen. Triterpenoid là một nhóm các hợp chất hóa học tự nhiên được tìm thấy trong nhiều loài động vật và thực vật [ 15 , 16 ] . Triterpenoid thực hiện nhiều chức năng khác nhau trong hóa học, đóng vai trò là tiền chất quan trọng trong quá trình sinh tổng hợp các hợp chất khác nhau như steroid và saponin [ 17 ]. Triterpenoid được coi là thành phần hoạt tính sinh học thiết yếu trong y học cổ truyền ở nhiều quốc gia, chẳng hạn như Trung Quốc, Nhật Bản, Hàn Quốc và Việt Nam [ 18 ]. Triterpenoid là các hợp chất được tìm thấy trong nhiều loại thực vật, thuộc nhóm isoprenoid. Các hợp chất này thường có trong thực vật dưới dạng glycoside và saponin. Do hoạt tính sinh học đa dạng và sự sẵn có dồi dào, triterpenoid được coi là nguồn nguyên liệu thô đầy hứa hẹn cho ngành công nghiệp dược phẩm [ 19 ].
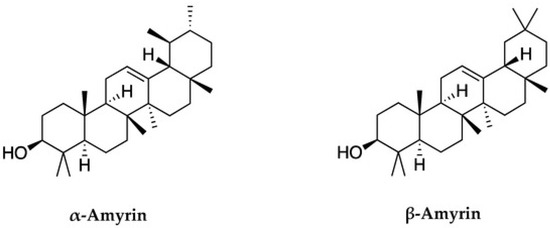
Trong nhóm axit triterpenoid, α-amyrin (đặc trưng bởi khung ursane) và β-amyrin (đặc trưng bởi khung oleanane) là hai hợp chất có cấu trúc liên quan, cả hai đều thuộc nhóm axit triterpenoid [ 20 ]. Trong số này, triterpen loại ursane, có nguồn gốc từ α-amyrin, là triterpen phổ biến nhất, trong khi triterpen loại oleanane có nguồn gốc từ β-amyrin [ 21 ]. Amyrin có trong lớp sáp bề mặt của quả cà chua và cà phê bồ công anh. Đặc biệt, hai hợp chất này đã được nghiên cứu rộng rãi và được các tác giả chiết xuất thành công với số lượng đáng kể (10,75 g/kg) từ lá của Celastrus hindsii [ 22 ].
α-Amyrin và β-amyrin là hai hợp chất tiêu biểu trong nhóm triterpenoid. Các hợp chất này có công thức hóa học tương tự nhau nhưng khác nhau về cấu trúc phân tử của dạng α và β. Trong α-amyrin, nhóm (CH3-29) được gắn vào vị trí carbon C19 hướng về phía trước, trong khi nhóm (CH3-30) được gắn vào vị trí carbon C20 hướng về phía sau. Trong β-amyrin, cả hai nhóm (CH3-29) và (CH3-30) đều được gắn vào vị trí carbon C20 và hướng về phía trước ( Hình 1 ) [ 20 , 21 , 23 ]. α-Amyrin và β-amyrin thường được tìm thấy trong thực vật, và nghiên cứu trước đây đã chiết xuất thành công chúng từ Celastrus hindsii . Tuy nhiên, giá trị dược phẩm của các hợp chất này chưa được biết đến rộng rãi, và việc phân lập chúng vẫn còn nhiều thách thức. Do đó, nghiên cứu này làm rõ tiềm năng điều trị và hoạt động sinh học của hai hợp chất này. Bản báo cáo này đóng vai trò như một bản tóm tắt toàn diện về giá trị của chúng, nhằm tối đa hóa tiềm năng vốn có của hỗn hợp này như một nền tảng cho việc phát triển các sản phẩm dược phẩm trong tương lai.
2. Materials and Methods
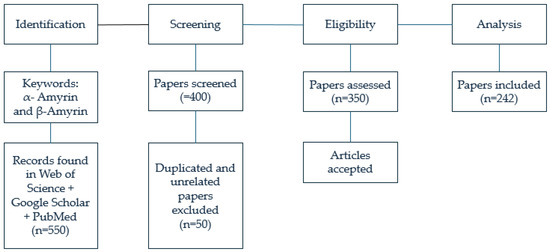
This study was carried out based on a comprehensive review of 550 scientific articles concerning α- and β-amyrins. Among these, 400 articles were screened and identified as relevant to the objectives of this research. From these, 350 articles with content most closely aligned with the research objectives were selected. Subsequently, 108 articles were excluded due to limitations in transparency, inadequate language clarity, or lack of indexing within the Science Citation Index (SCI) system. Ultimately, 242 articles containing the most comprehensive and clearly presented content and data were selected to serve as the foundation for this research project (). This article integrates knowledge from reputable global sources. The information and data were derived from authoritative indexing systems, including the Science Citation Index (SCI), Science Citation Index Expanded (SCIE), and the Institute for Scientific Information (ISI), all of which cover high-quality research published in a vast number of peer-reviewed journals worldwide. The foundation of this research is a rigorously selected database of 242 scientific articles sourced from reputable academic publishers, including Springer, Elsevier, Wiley-Blackwell, Taylor & Francis, Oxford University Press, Cambridge University Press, the University of Chicago Press, InderScience Publishers, and Edward Elgar Publishing, covering the period from 1999 to 2025. Furthermore, the study integrates the authors’ expertise, informed by two prior research publications in MDPI-indexed journals.
3. Results
3.1. Anti-Inflammatory Potential of α- and β-Amyrins
The anti-inflammatory properties of α- and β-amyrins were assessed in a rat model of acute periodontitis, in which the condition was induced by ligating the upper right second molar. Two hours prior to ligature placement, rats received intraperitoneal injections of α- and β-amyrins at doses of 5–10 mg/kg. Lumiracoxib and dexamethasone were used as positive controls, while a placebo group served as the negative control. Six hours after induction, plasma levels of tumor necrosis factor-α (TNF-α) were measured. After 24 h, the rats were sacrificed, and gingival tissues were analyzed using myeloperoxidase (MPO) activity and thiobarbituric acid reactive substance (TBARS) assays. The results showed that α- and β-amyrins, particularly at the 5 mg/kg dose, significantly reduced markers of inflammation. While lumiracoxib produced variable effects on the measured parameters, α- and β-amyrins demonstrated notable efficacy in mitigating acute inflammation. These results underscore the potential of α- and β-amyrins as promising therapeutic agents for managing inflammation associated with periodontal disease, and they highlight the need for further research into their efficacy in preventing chronic bone loss [
24].
The anti-inflammatory and analgesic effects of α- and β-amyrin were assessed in experimental models involving the activation of cannabinoid receptor type 1 (CB1) and type 2 (CB2) signaling pathways. The findings demonstrated that both compounds markedly inhibited the production of pro-inflammatory cytokines and downregulated the expression of nuclear factor-kappa B (NF-κB) binding protein and cyclooxygenase (COX) enzymes. These results indicated that α- and β-amyrins may exert their anti-inflammatory effects, at least partially, through modulation of the endocannabinoid system, thereby highlighting their potential as therapeutic candidates for the treatment of inflammation and pain-related disorders [
25].
The anti-inflammatory properties of α- and β-amyrin were evaluated in a TNBS-induced murine model of colitis, with the compounds administered in a 1:1 ratio. Following the induction of colitis via rectal administration of trinitrobenzene sulphonic acid (TNBS), the animals were monitored for 72 h to assess clinical and biochemical parameters. Systemic treatment with α- and β-amyrin (3 mg/kg, intraperitoneally) was compared to treatment with dexamethasone and to a vehicle-treated control group. Disease progression was evaluated through both macroscopic and microscopic assessments of colonic lesions, along with measurements of myeloperoxidase (MPO) activity and cytokine levels. Immunohistochemical analysis was performed to assess the expression of cyclooxygenase-2 (COX-2), vascular endothelial growth factor (VEGF), phosphorylated NF-κB (phospho-p65), and phosphorylated cAMP response element-binding protein (phospho-CREB). TNBS-induced colitis was characterized by severe tissue damage, marked neutrophil infiltration, and elevated levels of pro-inflammatory mediators. Treatment with α- and β-amyrin led to notable improvements in colonic tissue morphology, a significant reduction in polymorphonuclear cell infiltration, decreased levels of interleukin-1β (IL-1β), and restoration of the anti-inflammatory cytokine interleukin-10 (IL-10) in the colon. Furthermore, α- and β-amyrin markedly suppressed the expression of vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), phosphorylated NF-κB (phospho-p65), and phosphorylated CREB (phospho-CREB). These findings highlight their potent anti-inflammatory activity in TNBS-induced colitis and underscore their potential as therapeutic candidates for the treatment of inflammatory bowel disease (IBD) [
26].
α- and β-Amyrin exhibit potent anti-inflammatory activity, notably through modulation of the endocannabinoid system. In a murine model of colitis induced by dextran sulfate sodium (DSS), administration of these compounds significantly attenuated the severity of colonic lesions. This therapeutic effect was associated with decreased activity of inflammatory enzymes, including myeloperoxidase (MPO) and
N-acetylglucosaminidase. Administration of the triterpenes also significantly reduced levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and various chemokines, while enhancing the expression of the anti-inflammatory cytokine interleukin-4 (IL-4). Moreover, α- and β-amyrin downregulated the mRNA expression of several adhesion molecules involved in leukocyte recruitment and inflammation, including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), platelet endothelial cell adhesion molecule-1 (PECAM-1), β2-integrin, CD68, and P-selectin. Importantly, these compounds also inhibited cannabinoid receptor type 1 (CB1), suggesting a role in modulating cannabinoid-mediated signaling. Additionally, they suppressed the expression of endocannabinoid-degrading enzymes such as monoglyceride lipase (MGL) and fatty acid amide hydrolase (FAAH), thereby enhancing endocannabinoid tone. Collectively, these findings support the therapeutic potential of α- and β-amyrin as novel agents for the treatment of inflammatory diseases, particularly those involving dysregulation of the cannabinoid system [
27].
α- and β-amyrin have demonstrated considerable therapeutic potential in the treatment of acute pancreatitis. In a model of pancreatitis induced by L-arginine, treatment with these triterpenes significantly reduced the elevated pancreas wet weight to body weight ratio, an established marker of inflammation and edema. Furthermore, administration of α- and β-amyrin led to substantial decreases in serum amylase and lipase levels, which are key biochemical indicators of pancreatic injury. The compounds also markedly lowered concentrations of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). In pancreatic tissues, treatment with α- and β-amyrin reduced the activity of inflammation and oxidative stress markers, including myeloperoxidase (MPO), thiobarbituric acid reactive substances (TBARSs), and nitrate/nitrite levels. Immunohistochemical analysis further confirmed decreased expression of tumor necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS), reinforcing the observed anti-inflammatory effects. Collectively, these findings indicate that α- and β-amyrin exert both antioxidant and anti-inflammatory actions, highlighting their potential as therapeutic agents for the management of acute pancreatitis [
28].
3.2. Antidiabetes Potential of α- and β-Amyrins
α- and β-amyrins have demonstrated significant antidiabetic and hypolipidemic properties in experimental models. Oral administration of these compounds resulted in marked reductions in blood glucose levels, total cholesterol, and serum triglycerides. At a dose of 100 mg/kg, α- and β-amyrin not only normalized blood glucose levels but also exhibited strong lipid-lowering effects. During oral glucose tolerance tests, elevated glucose concentrations were significantly reduced, indicating improved glucose metabolism. Additionally, plasma insulin levels and histopathological analysis of pancreatic tissue supported the beneficial role of α- and β-amyrin in preserving pancreatic function. Rats administered α- and β-amyrin at doses of 10, 30, and 100 mg/kg demonstrated dose-dependent improvements in lipid profiles. Notably, treatment at 100 mg/kg led to a substantial decrease in very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) cholesterol levels, accompanied by an increase in high-density lipoprotein (HDL) cholesterol. These findings underscore the potential of α- and β-amyrin as therapeutic agents for managing hyperglycemia and dyslipidemia. Moreover, their efficacy in reducing risk factors associated with atherosclerosis suggests their promise as lead compounds in the development of novel treatments for diabetes and cardiovascular diseases [
29].
β-amyrin, extracted from the roots of
Hemidesmus indicus, has demonstrated notable antidiabetic activity in experimental models. Its derivative, β-amyrin palmitate, exhibited significant antihyperglycemic effects in glucose-loaded rats. Remarkably, this compound showed potent antidiabetic activity in both alloxan-induced and streptozotocin-induced diabetic rat models, even at a very low dose of 50 µg/kg body weight. The primary mechanism of action of β-amyrin palmitate appears to involve inhibition of intestinal glucose absorption, thereby reducing postprandial hyperglycemia. These results suggest that β-amyrin and its derivatives have strong potential as lead compounds for the development of future antidiabetic therapeutics [
30].
β-amyrin has been shown to possess significant antibacterial and antidiabetic properties through its enzymatic inhibitory activity. At a concentration of 24.24 µg/mL, β-amyrin effectively inhibited violacein production, with inhibit percentages ranging from 22.9 ± 1.2% to 42.1 ± 1.0%, suggesting its potential as an antiquorum sensing agent. Furthermore, β-amyrin exhibited notable α-amylase inhibitory activity, achieving inhibition rates between 49.8 ± 0.3% and 69.3 ± 1.0% at a concentration of 10 µg/mL. In addition, β-glucosidase inhibition assays further confirmed the compound’s effectiveness in targeting key carbohydrate-metabolizing enzymes. These findings highlight β-amyrin’s dual functionality in both antimicrobial and antidiabetic roles, supporting its potential development as a multifunctional therapeutic agent [
31].
α-Amyrin has demonstrated significant hypoglycemic activity in murine models and has been shown to exert dual effects on peroxisome proliferator-activated receptors (PPARδ and PPARγ) in 3T3-L1 adipocytes, highlighting its potential for the management of type 2 diabetes. Mechanistically, α-amyrin activates both PPARδ and PPARγ, along with adenosine monophosphate-activated protein kinase (AMPK) and protein kinase B (Akt). These signaling pathways are crucial for the translocation of glucose transporter 4 (GLUT4), which plays a pivotal role in enhancing insulin sensitivity and combating insulin resistance. Studies suggest that α-amyrin acts as an allosteric activator of AMPK, promoting GLUT4 translocation and improving glucose uptake in target tissues. Given these multiple mechanisms, α-amyrin is considered a promising bioactive molecule for the development of novel, multi-target therapeutic strategies aimed at addressing diabetes and its associated metabolic complications [
32].
β-Amyrin has been identified as a promising antidiabetic compound with beneficial effects on preventing renal failure. In vivo, streptozotocin-induced diabetic rats were used as a model for diabetic nephropathy (DN), while in vitro, high glucose (HG)-stimulated human proximal tubular HK-2 cells served as an experimental model. β-Amyrin demonstrated its ability to alleviate renal injury in diabetic rats and reduce both the inflammatory response and apoptosis in HG-stimulated HK-2 cells. Mechanistically, β-amyrin induced the upregulation of miR-181b-5p, which was found to interact with high mobility group box 2 (HMGB2), as confirmed by luciferase reporter assays. Furthermore, β-amyrin promoted the downregulation of HMGB2 expression. Overexpression of HMGB2 was shown to reverse the protective effects of miR-181b-5p on the inflammatory response and apoptosis of HG-treated HK-2 cells, suggesting that β-amyrin exerts its nonprotective effects through the miR-181b-5p/HMGB2 axis. These findings underscore β-amyrin’s potential as a therapeutic agent for managing diabetic nephropathy and preventing renal damage in diabetes [
33].
β-Amyrin has demonstrated significant antidiabetic potential, as evidenced by its α-amylase inhibitory activity in vitro, with an IC
50 of 19.50 µg, which is comparable to the standard antidiabetic drug acarbose (IC
50: 11.25 µg). Further computational studies revealed that β-amyrin exhibits high binding affinity to four key targets involved in diabetes regulation: glucagon-like peptide-1 (GLP-1), glycogen synthase kinase (GSK), glucokinase (GK), and insulin receptor tyrosine kinase (IRTK). Additionally, β-amyrin was found to possess favorable physicochemical properties, including high stability and bioactivity, a smaller energy gap, lower hardness, and higher softness, all of which contribute to its potential as a promising lead compound for antidiabetic drug development [
34].
3.3. Antiatherosclerosis Effect of α- and β-Amyrins
α- and β-Amyrins, isolated from a 95% ethanol extract of the fruits of the wild species
C. scabrifolia, were evaluated for their lipid-lowering potential. Comprehensive spectroscopic and biochemical analyses were conducted to characterize the compounds. Their lipid-lowering activity was assessed using an in vitro HepG2 cell model. Results from molecular docking studies further supported the findings, indicating that both α- and β-amyrins exhibit strong potential as lipid-lowering agents [
35].
α- and β-Amyrins were isolated from the stem and leaf extracts of
Rhus sylvestris Siebold. Spectroscopic analysis confirmed the identity of the compounds, and cytotoxicity assays indicated that they were non-toxic at concentrations ranging from 0 to 1.0 μM. The compounds were further evaluated for their immunomodulatory properties, particularly their ability to inhibit cytokine secretion. In vitro studies using the murine macrophage cell line RAW264.7 demonstrated that α- and β-amyrins significantly reduced lipopolysaccharide (LPS)-induced secretion of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). Notably, TNF-α secretion was inhibited even at a low concentration of 0.01 μM. These findings suggest that α- and β-amyrins hold promise as potential therapeutic agents for TNF-α-related inflammatory conditions, including transplant rejection, type II diabetes, and atherosclerosis [
36].
α- and β-Amyrins, isolated from
Protium heptaphyllum, have demonstrated significant antiatherosclerotic potential and hepatoprotective effects. Using a mouse model of non-alcoholic fatty liver disease (NAFLD), male Swiss mice were fed a high-fat diet (HFD) for 15 weeks to induce fatty liver pathology. Histological analysis of liver tissue sections using hematoxylin and eosin (H&E) staining, along with biochemical evaluations, revealed that administration of α- and β-amyrins significantly attenuated hepatic steatosis and inflammation. Further mechanistic insights were obtained through RT-qPCR and Western blotting, which showed that these compounds reversed the expression of key signaling pathways associated with lipid accumulation and inflammatory responses. These findings support the potential of α- and β-amyrins as therapeutic agents for the prevention and treatment of NAFLD [
37].
α- and β-Amyrins, isolated from
Euphorbia hirta L., have demonstrated potent anti-inflammatory effects, particularly in the context of vascular inflammation. In vitro assays were conducted using endothelial cells (SVEC4-10 cell line) treated with a medium composed of 50% lipopolysaccharide (LPS)-activated macrophage culture supernatant (RAW medium). Treatment with α- and β-amyrins significantly inhibited the expression of the endothelin-1 (ET-1) gene, a known pro-inflammatory and vasoconstrictive marker. Furthermore, α- and β-amyrins restored the mRNA expression of endothelial nitric oxide synthase (eNOS), which was otherwise suppressed by RAW medium exposure. These findings suggest that α- and β-amyrins may serve as effective therapeutic agents in the prevention of vascular disorders associated with chronic inflammation [
38].
The effects of α- and β-amyrins on angiogenesis and the underlying molecular mechanisms were investigated using cultured human umbilical vein endothelial cells (HUVECs). Treatment with α- and β-amyrins was found to be non-cytotoxic to HUVECs. These compounds promoted angiogenic activity by enhancing tube-like structure formation and increasing cell migration. Moreover, α- and β-amyrins significantly stimulated the phosphorylation of Akt and endothelial nitric oxide synthase (eNOS), resulting in elevated nitric oxide (NO) production. These results suggest that α- and β-amyrins promote neovascularization in endothelial cells through an Akt-eNOS signaling-dependent mechanism. Therefore, α- and β-amyrins may represent promising therapeutic agents for the treatment of vascular diseases [
39].
The effects of α- and β-amyrins (20 mg/kg for 15 days) on vascular reactivity were evaluated in a mouse model of diet-induced obesity. Mice were fed a high-fat diet (HFD) for 15 weeks to induce obesity. The contractile responses of isolated thoracic aorta to potassium chloride (KCl) and phenylephrine, as well as endothelium-dependent and -independent vasodilation induced by acetylcholine and sodium nitroprusside, respectively, were assessed. Treatment with α- and β-amyrins prevented HFD-induced vascular dysfunction by restoring the attenuated contractile response to phenylephrine and improving vasodilatory responses to both acetylcholine and sodium nitroprusside. Furthermore, α- and β-amyrin administration reversed impaired K
+ channel activation and restored the inhibitory effects of tetraethylammonium on vasodilation. These findings suggest that α- and β-amyrins exert significant vascular protective effects in the context of obesity-associated endothelial dysfunction [
40].
3.4. Antinociceptive Effect of α- and β-Amyrins
The analgesic activity of α- and β-amyrins, extracted from
Protium heptaphyllum, was evaluated using a murine model of oral pain induced by formalin or capsaicin. Mice were administered α- and β-amyrin intraperitoneally at doses of 10, 30, and 100 mg/kg. Morphine (5 mg/kg, subcutaneously) and vehicle (3% Tween 80) served as controls. Pain was induced by injecting 20 μL of formalin (1.5%) or capsaicin (1.5 g) into the orofacial region. α- and β-Amyrins significantly reduced facial rubbing behavior in both pain models. Notably, at 30 mg/kg, α- and β-amyrins potentiated the second phase of the formalin response in a naloxone-sensitive manner, indicating involvement of peripheral opioid pathways. In the capsaicin-induced pain model, α- and β-amyrins exhibited a pronounced analgesic effect at the 100 mg/kg dose. These findings suggest that α- and β-amyrins exert antinociceptive effects through mechanisms at least partially mediated by peripheral opioid receptors [
41].
The antinociceptive properties of α- and β-amyrins, extracted from
Protium kleinii, were evaluated in rat models of visceral and inflammatory pain. Visceral pain was induced by intravenous injection of acetic acid, and the compounds were administered via both intraperitoneal and oral routes. The treatment provided rapid and effective pain relief. Further, α- and β-amyrins were assessed in central nervous system pain pathways through formalin injections into the hind paw, examining nociceptive responses at the level of the cerebral cortex and ventricles. The compounds effectively inhibited both the neurogenic and inflammatory phases of nociception. Moreover, α- and β-amyrins significantly reduced nociceptive responses induced by 8-bromo-cAMP (8-Br-cAMP), 12-O-tetradecanoylphorbol-13-acetate (TPA), and glutamate, suggesting a broad-spectrum analgesic profile. Notably, these antinociceptive effects appeared to be independent of classical opioid, α-adrenergic, serotonergic, or nitrergic pathways. Furthermore, α- and β-amyrins significantly reduced mechanical hyperalgesia triggered by inflammatory mediators such as carrageenan, capsaicin, bradykinin, substance P, prostaglandin E2, 8-Br-cAMP, and TPA. These results suggest that α- and β-amyrins exert robust peripheral, spinal, and supraspinal antinociceptive effects, supporting their potential as novel agents for treating inflammatory and visceral pain [
42].
The analgesic potential of α- and β-amyrins was demonstrated in the acetic acid-induced writhing test, where these compounds exhibited significant activity in reducing nociceptive responses. When administered orally, α- and β-amyrins effectively decreased the number of abdominal constrictions induced by acetic acid, indicating strong peripheral analgesic properties. Furthermore, in the formalin-induced pain model, α- and β-amyrins attenuated the behavioral responses associated with both the neurogenic and inflammatory phases of pain. These findings underscored the role of α- and β-amyrins in modulating pain perception and support their potential as therapeutic agents for the management of inflammatory and visceral pain [
43].
Pharmacological activation of cannabinoid receptors CB1 and CB2 were a promising therapeutic strategy for the treatment of chronic pain and inflammation. In this context, α- and β-amyrins were investigated as bioactive compounds and demonstrated significant inhibition of persistent neuropathic pain and inflammation in murine models through oral administration. These triterpenes exerted their effects via both CB1 and CB2 receptor pathways. Notably, α- and β-amyrins exhibited strong inhibitory activity against the hydrolysis of 2-arachidonoyl glycerol (2-AG) in porcine brain homogenate, suggesting an indirect cannabimimetic mechanism. Rather than binding directly to cannabinoid receptors, α- and β-amyrins appear to enhance endocannabinoid signaling by preventing the enzymatic degradation of 2-AG. These findings position α- and β-amyrins as potential lead compounds for the development of novel analgesic and anti-inflammatory therapies targeting the endocannabinoid system [
44].
3.5. Antigout Effect of α- and β-Amyrins
α- and β-Amyrin extracted from
Celastrus hindsii were shown to possess antigout potential by inhibiting the activity of the xanthine oxidase (XO) enzyme, with an IC
50 value of 258.22 µg/mL [
23]. Compounds α- and β-amyrins, isolated from
Tabebuia roseoalba leaves and identified in ethanol extracts by HPLC analysis, were shown to reduce serum uric acid concentrations and inhibit the inflammatory process associated with gout. The study evaluated their antihyperuricemic, hepatic xanthine oxidoreductase inhibitory, and anti-inflammatory activities in hyperuricemic rats and monosodium urate crystal-induced paw edema models [
45]. β-Amyrin significantly reduced serum uric acid levels in hyperuricemic rats by inhibiting hepatic xanthine oxidase activity and effectively reduced monosodium urate crystal-induced paw edema. These findings suggest that β-amyrin is a promising agent for the treatment of gouty arthritis, hyperuricemia, and related inflammatory conditions [
46].
α- and β-Amyrin were isolated from the pericarp, heartwood, and seed of
Garcinia subelliptica. Structural identification of these triterpenoids was confirmed through spectroscopic analysis, including techniques such as NMR and mass spectrometry. Both compounds demonstrated a notable inhibitory effect on xanthine oxidase (XO), an enzyme involved in purine metabolism and a key contributor to uric acid production. This suggests their potential antigout and antioxidant applications. Flow cytometric analysis revealed that treatment of NTUB1 cells with compound 1, either alone or in combination with cisplatin, resulted in cell cycle arrest and a significant increase in apoptotic cell death after 24 h of exposure. These data suggested that the induction of cell cycle arrest and apoptosis in NTUB1 cells treated with compound 1 or with a combination of compound 1 and cisplatin for 24 h is mediated by an increased production of reactive oxygen species (ROS) [
47].
3.6. Positive Effects of α- and β-Amyrins on Nerves
α- and β-Amyrins were studied for their pharmacological effects on sleep in mice. The sleep was induced using pentobarbital, and the mice were then administered α- and β-amyrin at concentrations of 1, 3, or 10 mg/kg. The results showed that α- and β-amyrins significantly extended the sleep duration in mice. This suggests that α- and β-amyrins have potential anti-insomnia effects, likely through the activation of the GABAergic neurotransmitter system in the brain [
48]. α- and β-Amyrins isolated from
Protium heptaphyllum have demonstrated anticonvulsant, sedative, and anxiolytic properties. In the study, rats were treated with α- and β-amyrins at concentrations of 2.5, 5, 10, and 25 mg/kg (i.p. or orally). The results showed a significant increase in barbiturate-induced sleep duration, confirming the sedative effect. Additionally, the study found that tyrosine levels increased by 89%, while levels of GABA and glutamate decreased by 72%, 55%, and 60%, respectively. Furthermore, excitatory amino acids were reduced, and inhibitory amino acids were increased, suggesting a shift towards a more inhibitory neurotransmitter profile [
49].
α- and β-Amyrins extracted from
Protium heptaphyllum were evaluated for their ability to reduce capsaicin-induced analgesia in mice. The results demonstrated that orally administered α- and β-amyrins (doses ranging from 3 to 100 mg/kg) effectively inhibited pain sensation induced by capsaicin applied to the plantar (1.6 μg) and colon (149 μg). Notably, α- and β-amyrins did not alter sleep duration, nor did they impair walking, locomotion, or cause any observable abnormalities. Additionally, these compounds significantly blocked the hyperthermic response induced by capsaicin (10 mg/kg). These findings suggest that α- and β-amyrins may be potential compounds for analgesia, likely involving the vanilloid receptor (TRPV1) and opioid mechanisms [
50].
The isolation of α- and β-amyrin from
Lobelia inflata leaves was studied for its effects on the central nervous system in mice. The results showed that α- and β-amyrins significantly reduced the immobility time of mice in a dose-dependent manner (5, 10, and 20 mg/kg). These compounds also dose-dependently reduce locomotor activity and potentiated methamphetamine-induced locomotor antagonism. Unlike imipramine, α- and β-amyrins did not affect haloperidol-induced rigidity, tetrabenazine-induced ptosis, or apomorphine-induced stereotypy. They also had no effect on the 5-hydroxytryptophan-induced head-clonic reaction. Additionally, α- and β-amyrins (5, 10, and 20 mg/kg) had a stronger effect on pentobarbital sodium-induced delirium than imipramine (10, 20, and 40 mg/kg). These findings suggest that α- and β-amyrins exhibit sedative and antidepressant-like effects, comparable to the activity of meandering [
51].
α- and β-Amyrin, components found in the waxy surface of tomatoes and dandelion coffee, have been shown to improve memory impairment caused by cholinergic dysfunction. These compounds act as PI3K inhibitors, which help reduce long-term potentiation (LTP) impairment. In studies on Aβ-injected mouse models of Alzheimer’s disease, α- and β-amyrin were found to improve object recognition memory deficits. Furthermore, α- and β-amyrin treatment helped restore neurogenesis impairment induced by Aβ. These findings suggest that α- and β-amyrin are promising candidates for the treatment of Alzheimer’s disease [
52]. The isomer mixture of α- and β-amyrin found in the resin of
Protium heptaphyllum has demonstrated potential as an anti-inflammatory agent. Additionally, these compounds have been shown to have a protective effect on both the central and peripheral nervous systems, helping the brain respond to various effects on the central nervous system [
53].
3.7. Anti-Parkinsonian Effects of α- and β-Amyrins
α- and β-Amyrins have been shown to offer protective effects on dopaminergic neurons by reducing 6-hydroxydopamine (6-OHDA)-induced cell damage. These compounds exhibit strong antioxidant activity, reducing intracellular reactive oxygen species (ROS) in
C. elegans. α- and β-Amyrins significantly decrease α-synuclein aggregation in the NL5901 transgenic strain, upregulate LGG-1 mRNA expression, and increase the number of localized LGG-1 dots in the DA2123 transgenic strain. These results suggest that α- and β-amyrins could be beneficial in the treatment and slowing of Parkinson’s disease progression [
54]. The use of α- and β-amyrins has been explored as a potential treatment to prevent Parkinson’s disease by targeting misfolded proteins and damaged organelles. These effects have been tested through mechanisms related to LGG-1, suggesting that α- and β-amyrins may play a role in mitigating the underlying causes of Parkinson’s disease [
55]. Autophagy has been recognized as a promising therapeutic approach for preventing Parkinson’s disease, as it plays a crucial role in eliminating misfolded proteins and damaged organelles, which are key contributors to the disease’s progression [
56]. During the process of autophagosome formation, LGG-1 plays a crucial role in structural maintenance, making it a valuable marker for monitoring autophagic activity [
57].
α- and β-Amyrins have been shown to participate in the LGG-1-related autophagy pathway by enhancing LGG-1 expression and increasing the number of localized LGG-1 spots. These compounds have demonstrated the ability to protect dopaminergic neurons by reducing cell damage and preventing α-synuclein aggregation, both of which are associated with Parkinson’s disease symptoms [
58]. Anti-Parkinsonian activities are closely linked to the regulation of cholesterol metabolism, as sterol-mediated cholesterol metabolism plays a significant role in neurological diseases. Low levels of LDL-C are often associated with a higher incidence of Parkinson’s disease. α- and β-Amyrins have been shown to regulate cholesterol metabolism, supporting their potential antineurological and antidementia effects. This suggests that α- and β-amyrins could be promising agents for the treatment of Parkinson’s disease [
59].
3.8. Anticancer Potential of α- and β-Amyrins
Because of constant demand to develop new, effective and affordable anticancer drugs, the traditional medicinal system is a valuable and alternative resource for identifying novel anticancer agents. A study investigated the inhibitory activity of the compounds in methanolic bark extract of
Shorea robusta on hepatocellular carcinoma by molecular docking studies on isolated α- and β-amyrins compounds. These compounds are used for docking on human oncogene protein. Docking studies of designed compounds were carried out using molecular docking servers. The recorded for alpha and beta amyrin binding with human Ras protein was −9.36 kcal/mol and −8.90 kcal/mol, respectively. Frontier singly occupied molecular orbitals (SOMO) were studied by density functional theory (DFT) and time dependent-DFT calculations. Among the calculated band gap energies, the β-MOs of α- and β-amyrin compounds have a very narrow band gap (−1.057 eV). These findings explain the anticancer activities of alpha and beta amyrin from
Shorea robusta [
60].
Another study aimed to evaluate the chemical composition of
P. heptaphyllum resin and cytotoxicity on a breast cancer cell line (MCF-7). The chemical composition of the resin was determined by gas chromatography coupled with a mass spectrometer. Cytotoxicity was evaluated using an MTT assay. Annexin V-FITC, caspase-3, Angiotensin Converting Enzyme activity, and Tumor Necrosis Factor alpha (TNF-α) assays were performed to evaluate apoptosis and inflammatory events. The resin consisted of triterpenes, such as α- and β-amyrin. Cytotoxicity was only observed in fractions enriched with α- and β-amyrin. The resin and fractions elicited antiproliferative activity, increased activity of caspase-3 and ACE, and decreased the TNF-α level. Altogether, the resin and fractions enriched with α- and β-amyrin promoted cytotoxicity and apoptosis [
61].
α- and β-Amyrins have been studied to exhibit significant pharmacological properties. One study was conducted to investigate the anticancer and pro-apoptotic effects of β-amyrin against HepG2 liver cancer cells. The antiproliferative potential of α- and β-amyrins was evaluated using the MTT assay. Apoptosis was assessed through DAPI staining, and DNA damage was analyzed using the comet assay. Cell cycle analysis was performed using flow cytometry, and protein expression levels were assessed by Western blotting. β-Amyrin exhibited significant anticancer activity against Hep-G2 liver cancer cells, with an IC
50 value of 25 µM. The anticancer effects of α- and β-amyrins were attributed to the induction of apoptosis and G2/M phase cell cycle arrest in a dose-dependent manner. Based on the results of the study, α- and β-amyrins may represent a promising lead compound for the treatment of liver cancer [
62].
The anticancer activity of α- and β-amyrins have been evaluated in various therapeutic combinations against colon cancer, aiming to identify the key mechanisms involved in mitigating nickel-induced carcinogenesis. To evaluate the ligand–protein interactions of four selected compounds with Vascular Endothelial Growth Factor (VEGF), Matrix Metalloproteinase-9 (MMP-9), and Interleukin-10 (IL-10), a molecular docking approach was employed using the PyRx bioinformatics tool. Ten rats, each weighing between 160 and 200 g, were administered intraperitoneal injections of nickel at a dose of 1 mL/kg once per week for three months. Following this exposure, the rats were treated with β-amyrin at a dose of 100 mg/kg body weight for one month. Correlation analysis was performed using Pearson’s correlation matrix. All parameters were significantly elevated in the positive control group, indicating pronounced inflammation. The study concluded that α- and β-amyrins are potent anticancer agents capable of targeting cancer biomarkers and hold future promise as a superior therapeutic approach against colon cancer [
63].
Natural α- and β-amyrins were isolated from the endemic Brazilian plant
Esenbeckia grandiflora Mart., and eight synthetic derivatives were subsequently obtained through esterification with bromoacetate, followed by treatment with various amines. The structures of all compounds were confirmed through analysis of
1H and
13C nuclear magnetic resonance (NMR), Fourier-transform infrared spectroscopy (FTIR), and high-resolution mass spectrometry (HRMS) data. The synthetic derivatives were evaluated for their cytotoxic activity against human tumor cell lines, including PC3 (prostate carcinoma), HCT-116 (colon carcinoma), and HL60 (leukemia). The HCT-116 and PC3 cell lines exhibited weak tumor growth inhibition, with inhibition ranges of 13.9–25.4% and 10.3–28.8%, respectively. In contrast, the derivatives demonstrated moderate cytotoxic activity against the HL60 leukemia cell line, with inhibition ranging from 13.6% to 59.0% [
64].
α- and β-Amyrins were found to be highly active and non-toxic compounds against tumor cells. Their inhibitory effects were tested on four human tumor cell lines (HL-60, MDAMB-435, SF-295, and HCT-8), as well as on normal peripheral blood mononuclear cells (PBMCs). The results demonstrated that α- and β-amyrins exhibited significant anticancer activity while showing minimal toxicity to normal cells, making them promising candidates for further exploration in cancer therapy [
65].
HeLa cells were treated with β-amyrin, and the cells were pretreated with 100 µM
N-acetyl-L-cysteine for 1 h before treatment. The efficacy of β-amyrin was evaluated through antiproliferative activity measured by the MTT assay, genotoxicity using the micronucleus assay, and the determination of reactive oxygen species (ROS), nitric oxide (NO), and caspase-3 levels using fluorescence spectrophotometry and a colorimeter. Protein expression was assessed via immunoblotting. β-Amyrin (10–200 µM) inhibited the growth of cancer cells, with IC
50 values ranging from 10 to 100 µM. Western blot analysis revealed that β-amyrin induced apoptosis-related proteins, including Bcl-2, caspase-3, caspase-9, phospho-p38 mitogen-activated protein kinase (MAPK), and phospho-Jun N-terminal kinase (JNK) in cancer cells. Genotoxic effects were observed following β-amyrin treatment, and HeLa cells exhibited a significant increase in total ROS. Protein expression analysis showed that β-amyrin upregulated MAPK-p38, phospho-JNK, and growth arrest and DNA damage-inducible β (GADD45β) in HeLa cells. These results suggest that β-amyrin induces apoptosis through a ROS-mediated mechanism by activating p38 MAPK and JNK pathways via the transcription factor GADD45β, which plays a major role in suppressing the growth of cervical cancer cells [
66].
The mixture of α-amyrin and β-amyrin demonstrated anticancer potential against the MCF-7 breast cancer cell line with an IC
50 value of 28.45 μg/mL. Importantly, this compound was not cytotoxic to normal cells, suggesting a selective action against cancer cells. These findings position α- and β-amyrin as promising candidates for developing treatments for breast cancer, with potential for fewer side effects due to their selective cytotoxicity [
67].
The mixture of α-amyrin and β-amyrin, isolated from dichloromethane extract, exhibited significant antitumor activity against several cancer cell lines, including KB-oral, and NCI-H187, with IC
50 values of 18.01 and 18.42 μg/mL, respectively. Additionally, α-amyrin and β-amyrin demonstrated strong antibacterial activity against
Escherichia coli, with a minimum inhibitory concentration (MIC) of 31.25 μg/mL. These findings highlight the dual potential of α- and β-amyrin as both anticancer and antibacterial agents [
68,
69].
The study on α-amyrin and β-amyrin from
Mesua ferrea stem bark demonstrated their significant in vitro anticancer activity against human colon cancer HCT116 cells. The compounds were tested using the MTT assay, which revealed their toxicity against various cancer cell lines, while also indicating their selective action, as they did not show similar toxicity to normal cell lines. This further supports the anticancer potential of α-amyrin and β-amyrin as promising therapeutic agents for cancer treatment [
70].
α-Amyrin- and β-amyrin-loaded nanocapsules for intestinal delivery were evaluated, preliminarily, for their cytotoxic ability against leukemic cells. Nanocapsule formulations were designed by the solvent displacement–evaporation method. The cytotoxic potential of the nanocapsules was evaluated in vitro using different leukemic lineages. Nanocapsules coated with Kollicoat
® Mae 100 P presented the smallest particle size (130 nm), the lowest zeta-potential (−38 mV), and the narrowest size distribution (PdI = 0.100). The entrapment efficiency was 65.47%, while the loading capacity was 2.40%. Nanocapsules release 100% of α-amyrin in 40 min (pH 7.4) by using a possible mechanism of swelling–diffusion. The formulation showed excellent on-shelf physicochemical stability for one year. Additionally, nanocapsules produced a selective cytotoxic effect on a human leukemia lineage Kasumi-1, an acute myeloid leukemia cell line, and produced cell death by apoptosis. α-Amyrin-loaded nanocapsules appear to be a promising nanoformulation that could be used against leukemia [
71].
3.9. Antibacterial Potential of α- and β-Amyrins
The mixture of α- and β-amyrins isolated from
Protium heptaphyllum demonstrates significant antibacterial activity against
Escherichia coli and
Staphylococcus aureus strains, including multidrug-resistant strains. Additionally, these compounds effectively inhibit efflux resistance mechanisms in
S. aureus strains 1199B and K2068, which carry the NorA and MepA efflux pumps. α- and β-Amyrins showed a higher affinity for these efflux pumps (NorA and MepA) compared to commonly used antibiotics like ciprofloxacin and norfloxacin. This suggests that α- and β-amyrins may enhance antibiotic activity by inhibiting efflux pumps, thus preventing bacteria from expelling the antibiotics. These findings support the potential of α- and β-amyrins as promising antibacterial agents, especially when combined with conventional antibiotics, to combat multidrug-resistant infections [
72].
A study demonstrated that the mixture of α- and β-amyrin isolated from
Morinda lucida leaves exhibited strong antibacterial activity against multidrug-resistant strains of Enterobacteriaceae, including
Klebsiella,
Pragia,
Serratia,
Enterobacter,
Providencia, and
E. coli. The compound mixture showed inhibition zones ranging from 15 to 18 mm at a concentration of 0.093 µg/mL. These results suggest that α- and β-amyrins from
M. lucida could be potent agents for treating infections caused by antibiotic-resistant bacteria. This supports the traditional use of
M. lucida in herbal medicine, and these compounds may offer alternative or complementary treatment for infectious diseases that are resistant to conventional antibiotics [
73].
Another study demonstrated that the α- and β-amyrin fraction (3.4 mg/mL) from
C. trichotomum exhibited significant antibacterial activity against
E. coli,
S. aureus, and
H. pylori. The compounds showed inhibition zones of 12 mm against
E. coli and 13 mm against
H. pylori, indicating their potential as antibacterial agents. At a lower concentration of 1.7 mg/mL, α- and β-amyrins still showed inhibition against
H. pylori, with inhibition zones of 10 mm and 11 mm, respectively. These findings suggest that α- and β-amyrins from
C. trichotomum could serve as promising therapeutic agents against infections caused by these bacterial strains, offering a potential alternative to traditional antibiotics [
74].
3.10. Anti-HIV Potential of α- and β-Amyrins
The potential of α- and β-amyrins in combating HIV has been studied with promising outcomes. These compounds were extracted from the stems and roots of
Kadsura lancilimba. Their structures and stereo chemistries were identified primarily from mass and NMR spectral data. α- and β-Amyrins inhibited HIV replication with an (IC
50: 1.4 μg/mL). α- and β-Amyrins have shown antiviral activity, particularly against HIV, making them a significant discovery in the pursuit of treatments for this “disease of the century.” Their therapeutic potential could be a step forward in developing alternative treatments for HIV, especially given the ongoing need for more effective antiviral drugs [
75]. This underscores the urgent need for safer, more effective drugs to combat resistant strains and advance acquired immunodeficiency syndrome (AIDS) therapeutics. α- and β-Amyrins were identified from
Uncaria rhynchophylla hooks. These compounds exhibited potent inhibition of HIV-1 protease (PR), one of the essential enzymes in the virus’s life cycle, with 3β-hydroxy-27-p-Z-coumaroyloxyurs-12-en-28-oic acid (8) showing the most potent inhibitory activity.In a study using in silico docking, triterpene ester 8 demonstrated conventional hydrogen bonding with specific amino acid residues, Asp29B, Lys45B, and Asn25A, interacting with the aromatic hydroxyl group at position 7 and the carboxylic acid at position 28. Additionally, these interactions occur via π–anion and π–alkyl and alkyl hydrophobic interactions, which are responsible for the compound’s mode of action. These molecular docking studies strongly confirmed an excellent SAR. The study suggests that triterpene esters from
U. rhynchophylla could represent a new class of potent HIV-1 PR inhibitors with less toxicity, suitable for combination antiretroviral therapy for AIDS [
76]. The isolation of α- and β-amyrins, along with 10 other triterpene compounds from dried stems of
Stauntonia obovatifoliola Hayata subsp, represented a significant step in the search for potential anti-HIV agents. Through the analysis of high-resolution EI/FAB-MS spectral data and 1D and 2D NMR spectra, the structures of these compounds were elucidated. Notably, α- and β-amyrins, along with other compounds from this plant, demonstrated HIV-1 protease inhibitory activity in the studies. This discovery highlighted the potential of these triterpenes as promising candidates for the development of novel anti-HIV therapies. Further, these results emphasize their importance in addressing the ongoing global HIV/AIDS epidemic [
77]. α- and β-Amyrins, isolated from the leaves and twigs of
Gardenia carinata, were structurally characterized using spectroscopic methods. These triterpenoids exhibited notable biological activities, specifically antitopoisomerase IIα and anti-HIV-1 properties. Remarkably, α- and β-amyrins were found to be completely non-cytotoxic, indicating their potential as safe therapeutic agents. The ability of these compounds to inhibit topoisomerase IIα, an enzyme critical in DNA replication and transcription, alongside their anti-HIV-1 activity, positions them as promising candidates for further research in the development of novel treatments for cancer and HIV, with minimal toxicity [
78].
α- and β-Amyrins are triterpenoids that were isolated from
Cassine xylocarpa stem and
Maytenus cuzcoina root bark and studied for their anti-HIV potential. The structures of these compounds were elucidated using 1D and 2D NMR techniques. To enhance their anti-HIV activity, derivatives of triterpenoids were synthesized through chemical modification of parent compounds. These modified derivatives exhibited inhibitory effects on HIV replication, with IC
50 values (4.08, 4.18, 1.70 μM) in the micromolar range. This indicates their strong potential as anti-HIV agents. The findings support the idea that α- and β-amyrins, both in their natural form and as modified derivatives, could be developed as valuable therapeutic agents for the treatment of HIV [
79]. α- and β-Amyrin derivatives have demonstrated potent anti-HIV activity, with the derivatives being specifically characterized based on their C-3 configuration. This study aimed to explore how modifications to the C-3 position and the overall triterpene backbone influence their anti-HIV effectiveness. The results provided valuable insights into the structure–activity relationship of α- and β-amyrins, highlighting how changes in the chemical structure can enhance their ability to inhibit the growth of HIV cells. This research further identifies α- and β-amyrins, particularly their modified derivatives, as promising candidates for the development of anti-HIV therapies. The study emphasizes the importance of structural modifications in improving the efficacy of natural compounds in combating complex diseases like HIV [
80].
3.11. The Isolation α- and β-Amyrins
The extraction of α- and β-amyrins from
C. hindsii has proven highly effective, yielding a remarkable amount of 10.75 g/kg dry weight. This highlighted
C. hindsii as a valuable source of raw material for α- and β-amyrins extraction. The identification and structural elucidation of the isolated compounds were carried out using advanced techniques such as gas chromatography–mass spectrometry (GC-MS), electrospray ionization–mass spectrometry (ESI-MS), and nuclear magnetic resonance (NMR). These methods enabled accurate characterization of the compounds and confirmed the effectiveness of the extraction process, thereby reinforcing
C. hindsii as a promising source of bioactive triterpenoids [
23].
4. Discussion
The world is currently facing a significant burden of disease. According to statistics from the World Health Organization, the global demand for pharmaceuticals increased steadily at an annual rate of 5.8% between 2014 and 2017 [
81]. The demand for pharmaceuticals is expected to increase steadily at an annual rate of 9 to 11.6% in the coming years [
82]. Antibiotic consumption accounted for the largest share, increasing by 16.3% from 29.5 to 34.3 billion defined daily doses (DDDs) between 2016 and 2023 [
83]. Cardiovascular drugs rank second, followed by anticancer drugs in third place and anti-infectives in fourth. The fifth largest segment of the pharmaceutical market includes treatments for metabolic disorders such as diabetes, together with thyroid and pituitary diseases [
84]. The demand for cholesterol-lowering drugs increased nearly fourfold, the use of antidepressants doubled, and the consumption of antihypertensives and antidiabetics nearly doubled between 1999 and 2012 [
85,
86]. In recent years, medications for the treatment of diabetes, hypertension, acquired immunodeficiency syndrome (AIDS), malaria, and tuberculosis have ranked among those with the highest global demand [
87]. The demand for pharmaceuticals is increasing not only in quantity but also in the need for higher quality and greater variety. In particular, the COVID-19 pandemic in 2020 starkly highlighted the urgent need for effective pharmaceuticals [
88]. Pharmaceutical consumption is increasing rapidly worldwide due to an aging population and the growing need for clinical management [
89]. Global population growth is a major factor contributing to the increased demand for pharmaceuticals [
90]. As society develops, the demand for pharmaceuticals, healthcare, and protection is increasingly focused on addressing more complex needs [
91]. Therefore, the pharmaceutical industry is becoming increasingly vital, and natural compounds continue to serve as a crucial source of raw materials for drug development [
92]. The discovery of natural compounds with potent biological activity has opened promising avenues for the development of new drugs [
93]. The pharmaceutical industry always requires strict standards for all input materials to ensure safety, efficacy, and quality in the development of therapeutic products [
94]. Therefore, the research and development of compounds such as α- and β-amyrin to serve the global pharmaceutical industry is of great importance.
illustrates the synthesis of α- and β-amyrin activities that have been studied, aiming to clarify the potential contributions of these compounds to the pharmaceutical industry. The pharmacological activities of the α- and β-amyrin mixture have been extensively studied both in vitro and in vivo to support future medicinal applications. Research has demonstrated that α- and β-amyrin possess a wide range of therapeutic properties, including analgesic, anti-inflammatory, anticonvulsant, antidepressant, gastroprotective, hepatoprotective, antipancreatitis, antihyperglycemic, and hypolipidemic effects [
95]. α- and β-Amyrin have also been shown to exhibit anticancer and anti-inflammatory properties, and beneficial effects in cardiovascular disease, diabetes, and arthritis [
96]. Notably, these compounds have been shown to be non-toxic to normal cells [
97]. Therefore, α- and β-amyrin represented a promising source of raw materials for the development of future medicinal products.
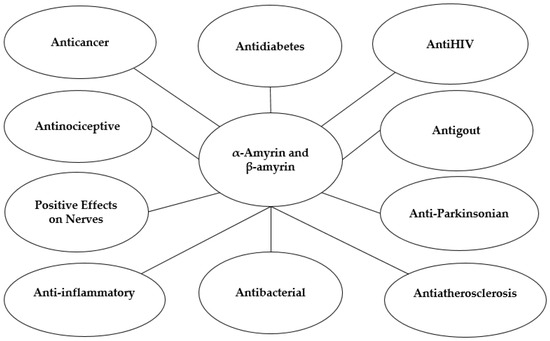
Inflammation is the body’s biological response to external or internal agents [
98]. Dangerous inflammatory conditions include hepatitis, appendicitis, colitis, gastritis, encephalitis, and nephritis [
99]. One of the most serious inflammatory diseases is pneumonia, which is closely linked to coronavirus disease [
100]. Periodontal disease leads to gum recession and deterioration of the bone structure that supports teeth [
101]. Inflammation can negatively affect bone health by disrupting bone growth and accelerating bone loss [
102]. Inflammation is also associated with depression, contributing to symptoms such as mood swings, loss of appetite, and sleep disturbances [
103]. Inflammation can activate immune cells to respond to bacteria already present in the digestive system, leading to chronic inflammation, inflammatory bowel disease (IBD), Crohn’s disease, and ulcerative colitis [
104]. Arthritis is associated with inflammation and an increased risk of cardiovascular problems [
105]. Inflammation is also associated with cardiovascular disease leading to atherosclerosis in the arteries, increasing the risk of cardiovascular disease [
106]. Chronic inflammation is associated with an increased risk of many types of cancer, including lung, esophageal, cervical, and gastrointestinal cancers. When immune cells initiate an inflammatory response, the regulatory ability of the immune system is impaired, creating a favorable environment for cancer cells to thrive [
107,
108]. Inflammation can negatively affect sleep, causing difficulty falling asleep or leading to excessive sleepiness [
109]. Therefore, inflammation is not only the body’s response to harmful agents, but also at the core of many serious diseases. This study demonstrated that the anti-inflammatory potential of α- and β-amyrin () has opened many opportunities for the development of adjuvant drugs for the treatment of related conditions.





